General
The Cochlear and Other Auditory Implants Centre is a part of the ENT Clinic at Nicosia General Hospital. The centre offers specialised medical and audiological services related to hearing implants for infants, children, and adults with hearing loss. The services include the audiological assessment of candidate patients for implantation, the surgical procedure for implant placement, postoperative care, as well as long-term monitoring and rehabilitation.
Hearing implants (cochlear, osseointegrated, middle ear implants) are small electronic devices that are surgically placed in the ear by a specialised ENT surgeon. They are recommended for individuals with severe hearing loss or deafness, for whom conventional methods (e.g., hearing aids) failed to restore hearing at satisfactory levels. After the procedure, the implant is activated and properly parametrised by a specialised audiologist. The process enables these patients to regain hearing and consequently improving their quality of life.
The Cochlear and Other Auditory Implants Centre is the only organised ENT centre in Cyprus that offers such services. Its operation is based on modern, international guidelines and protocols. From the initial visit of the hearing-impaired patient to the surgical procedure, postoperative care, and rehabilitation, specific protocols and procedures are followed rigorously to provide high-quality healthcare. Furthermore, the established collaboration of the centre with other healthcare and educational institutions in our country (e.g. Makarios Hospital, School for the Deaf) as well as corresponding centres abroad, is enabling and beneficial for the interdisciplinary approach needed for the effective treatment of individuals with hearing loss.
COCHLEAR IMPLANT
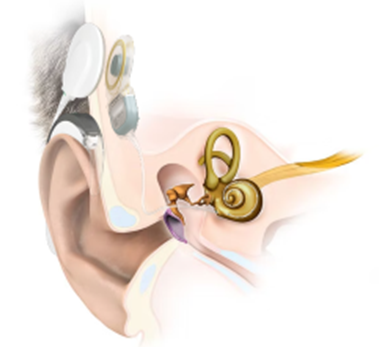
A cochlear implant is an electronic device that emits sound to individuals with severe to profound hearing loss. The cochlear implant bypasses the damaged structures of the inner ear (cochlea) and directly stimulates the auditory nerve. Unlike a common hearing aid, which amplifies the incoming sound into the ear, the cochlear implant captures the sound, transforms it into electrical stimuli, and stimulates the auditory nerve directly, offering effectively the sensation of hearing. Essentially, it is a bionic ear.
Learn more in the brochure below:
A cochlear implant consists of an external and internal component.
The external component: Sound and Speech Processor
This part of the system is worn behind the ear. It includes a microphone for sound detection, a battery as a power source, a processor, and a transmitter. It processes and digitises the sound signals and transmits them to the internal component through the transmitter.
The internal component: Receiver-Stimulator
The internal part of the system is surgically placed under the skin behind the ear. It consists of a magnet, an electronic system, and an intracochlear electrode array contained within a common cable. It receives signals from the transmitter of the external component, converts them into electrical signals, and transmits the information to the electrodes. The electrode array, which is positioned in the cochlea, stimulates the auditory nerve, which in turn transmits the information to the brain.
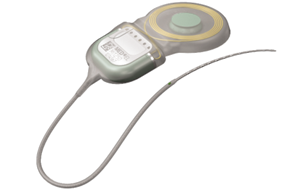
| COCHLEAR IMPLANT SYSTEM – MEDEL COMPANY |
External Component
Internal Component


| COCHLEAR IMPLANT SYSTEM – COCHLEAR COMPANY |
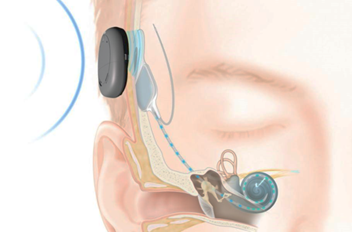
External Component
Internal Component



POSTOPERATIVE HEARING REHABILITATION
On its own, the surgical placement of the cochlear implant cannot restore hearing. In other words, the implant alone provides sounds in the form of electrical stimuli but the individual is not in position to interpret these stimuli, especially speech. To achieve this, the individual needs to undergo the process of auditory rehabilitation.
The purpose of postoperative rehabilitation is to train the brain to adapt to the sounds transmitted through the cochlear implant. This process involves two main parts: device programming – so that the individual can hear sounds through the implant, and rehabilitation – so that the individual can ‘make sense’ of the sounds they hear. The rehabilitation process can take several months. Improvement is noticeable from the beginning, and the ultimate goal is for the individual to be able to comprehend speech, even in challenging auditory environments (e.g., with background noise), or even to enjoy music.
The rehabilitation team consists of audiologists, speech-language pathologists, and educators for the deaf. The collaboration between the individual, their family, the rehabilitation team, and other healthcare and education professionals involved, sets the conditions for the successful outcome of the implantation.
RISKS AND POSSIBLE COMPLICATIONS OF COCHLEAR IMPLANTATION
Cochlear implant surgery is a safe surgical procedure with minimal complication rates. Particularly in specialised centres like ours, with high levels of expertise and extensive experience, complications of the procedure are rare. However, it is both an ethical and legal obligation of the doctors to inform the patient about the potential complications of the procedure, regardless of how rare they may be.
General complications: Bleeding, swelling, pain, wound healing disturbance or delay, aesthetically unsatisfactory outcome (e.g., scars), chronic discomfort at the implant site.
Specific complications: Ear inflammation(otitis/mastoiditis), seroma, ruptured eardrum, tinnitus, dizziness, pain at the implant site, changes in the aesthetics or position of the earlobe, meningitis, cerebrospinal fluid leakage, facial nerve palsy, stimulation of the facial nerve by the implant, taste disorders, injury to major blood vessels, haematoma or seroma, skin necrosis with exposed implant, extrusion of the electrode array, dislocation of the implant, difficulties and restrictions due to the implanted magnet (e.g., in undergoing magnetic resonance imaging), defects/faults/infections of the implant, need for replacement follow-up surgery, complete loss of any residual hearing, unsatisfactory implant function, non-use of the implant.
POSTOPERATIVE CARE INSTRUCTIONS AFTER COCHLEAR IMPLANTATION
These instructions apply for a period of 2 weeks after the surgery.
Keep the ear and the implant area dry and clean. Avoid activities that may increase pressure in the head area (e.g., intense physical activity, bending forward, straining during bowel movements, and lifting heavy objects). Avoid actions that may increase pressure in the nose (e.g., blowing the nose, sniffing with closed mouth and nose, air travel). Perform thorough daily care of the incision site and the implant area using aseptic techniques and antiseptic, according to the surgeon’s instructions.
Medication
Upon discharge, continue the prescribed medication at home. The treatment is usually administered for a total of 7 days, including the period of treatment within the hospital.
WARNING:
In cases where you notice:
ENT Outpatient Clinic
Monday-Friday, 08:00-15:00
ENT Inpatient Department
22603466 (Any day, any time)
RADIOLOGICAL EXAMINATIONS IN INDIVIDUALS WITH COCHLEAR IMPLANTS
Individuals with cochlear implants can undergo regular X-rays and computed tomography (CT) scans without restrictions.
Magnetic resonance imaging (MRI) has specific considerations. The strong magnetic field of an MRI machine can affect medical implants that contain magnets, such as the cochlear implant. The external part of the cochlear implant system is not safe for use during an MRI and must be removed before the procedure.
The internal part of the implant may move or rotate during the MRI examination, causing discomfort, pain, or injury. Additionally, the radio waves of the MRI machine can generate heat in the implant, leading to a sense of burning in the area. Individuals with cochlear implants need to be aware of these risks and ensure that the personnel conducting the MRI are informed about their implant such that appropriate precautions can be taken. Each cochlear implant model requires specific handling when undergoing MRI. Modern implants are compatible with MRI, meaning that the patient can undergo the examination with certain precautions. It should be noted that the magnet in the cochlear implant does not allow imaging of the structures of the head and brain in the area around the implant site. In special cases, prior to MRI, the removal of the magnet from the internal part of the implant may be necessary through a minor procedure under local anaesthesia. Also under local anaesthesia, a new magnet can be reinserted into the implant after the examination. The Cochlear and Other Auditory Implants Centre provides each patient with specific information about their implant, the possibility of undergoing an MRI examination, and the necessary precautions before and during the MRI.
ACCESSIBILITY TO SUITABLE INFRASTRUCTURES
(a) Audiological: Pure Tone Audiometry (PTA), in children play audiometry and Visual Reinforcement Audiometry (VRA), Otoacoustic Emissions (OAE), Auditory Brainstem Response (ABR), Cochlear implant speech processor programming.
(b) Vestibular system: Videonystagmography (VNG), posturography, video Head Impulse Test (vHIT), vestibular evoked myogenic potentials (VEMPs).
(c) Pediatric examinations (cardiological, ophthalmological, neurological).
(d) Genetic testing.
(e) Imaging examinations of computed tomography (CT) and high-resolution magnetic resonance imaging (MRI).
The Center ensures the performance of these specialized diagnostic tests through the provision of laboratories on its premises or in collaboration with other structures – laboratories in Cyprus, within or outside OKYPY: For (a) at the Audiology Laboratory of the ENT Clinic of the General Hospital of Nicosia, for (b) at the Vertigo Laboratory of the ENT Clinic of the General Hospital of Nicosia and the Cyprus Institute of Neurology and Genetics – Department of Neurobiology, for (c) at the corresponding departments of the Makarios Hospital – OKYPY, for (d) at the Clinical Genetics of the Makarios Hospital and other Departments of Medical Genetics, such as the Cyprus Institute of Neurology and Genetics – Department of Genetics, and for (e) at the Radiological Department of the General Hospital of Nicosia (high resolution CT scan, 1.5 Tesla MRI) and private radiological centers that have additional high-resolution MRI.
Related scientific publications from the Center